Who invented the recipe for smokeless powder. A brief history of the development of gunpowders. Loading Magnum cartridges
(English) Poudre B). They are classified into single-base, double-base and tri-base.
Encyclopedic YouTube
1 / 2
✪ What is the difference between black powder and smokeless powder?
✪ Demonstration experience "Smokeless powder"
Subtitles
Description
Smokeless powder burns only on the surface of granules, flakes or cylinders - for short, granules. Larger granules burn more slowly and the rate of their combustion is also controlled by a special coating that interferes with combustion, the main function of which is to regulate a more or less constant pressure on the rotating bullet or projectile, which has not yet left the gun barrel, which allows them to reach maximum speed.
In 1895-1896, “Morskoy Sbornik” published two large articles by D. I. Mendeleev under the general title “On pyrocollodium smokeless gunpowder,” which specifically examines the chemistry of the technology and describes the reaction for producing pyrocollodium. The volume of gases released during its combustion is characterized, and the raw materials are examined consistently and in detail. D.I. Mendeleev, scrupulously comparing pyrocollodion powder with other gunpowders in 12 parameters, demonstrates its undeniable advantages, first of all, composition stability, homogeneity, and the absence of “detonation traces.”
Gelatin powder
Application
Nowadays, propellants based only on nitrocellulose are known as monobase, while cordite-like ones are known as dibase. Tri-base cordites (Cordite N and NQ) with the addition of nitroguanidine were also developed, initially used in the large guns of naval warships, but also found their use in tank forces, and are now used in field artillery. The main advantage of three-base powders, compared to di-base ones, is the significantly lower temperature of the powder gases with similar efficiency. Prospects for the further use of gunpowders containing nitroguanidine are associated with small-caliber aviation and anti-aircraft guns that have a high rate of fire.
Smokeless gunpowder allowed the birth of modern semi-automatic and automatic weapons. Black powder left a large amount of solid products (40-50% of the mass of gunpowder) in gun barrels. The main solid combustion products of black powder, polysulfides (K2Sn, where n=2-6) and potassium sulfide (K2S), attract moisture and hydrolyze to potassium alkali and hydrogen sulfide. When smokeless powders burn, no more than 0.1 - 0.5% of solid products are formed, which made it possible to automatically reload weapons using many moving parts. It is worth considering that the combustion products of all smokeless powders contain a lot of nitrogen oxides, which increases their corrosive effect on the metal of the weapon.
Single- and double-base smokeless powders now make up the bulk of propellant explosives used in small arms. They are so common that most uses of the word "powder" refer specifically to smokeless powder, particularly when referring to handguns and artillery. Black powders are used as a propellant only in under-barrel grenade launchers, flare guns and some shotgun cartridges.
In some cases, for example, in a number of homemade hand grenades and improvised artillery shells, smokeless powder can also be used as a high explosive, for which the charging density is adjusted to a value corresponding to detonation, and powerful detonators are used. Unlike many explosives, a detonator cap is not required to use smokeless powder; any igniter is sufficient. The effectiveness of using smokeless powders as explosive explosives, in the event of ignition, is comparable to the effectiveness of using smokeless mine powder. When using powerful detonators (in practice, at least 400-600 grams of TNT), the efficiency is at the level of most individual explosive explosives.
Instability and stabilization
Nitrocellulose decomposes over time, releasing nitrogen oxides, which catalyze further breakdown of gunpowder components. During the process of decomposition reactions, heat is released, which, in the case of long-term storage of large quantities of gunpowder or storage of gunpowder at high temperatures (in practice, above 25 * C), may be sufficient for self-ignition.
Single base nitrocellulose propellants are most susceptible to decomposition; dibasic and tribasic decompose more slowly, which is associated with a higher content of chemical resistance stabilizers and their more uniform distribution in the volume of gunpowder, since nitroglycerin and other plasticizers help transform nitrocellulose into a homogeneous plastic state. Acidic chemical decomposition products (mainly nitrogen oxides, nitrous and nitric acids) of the energy-rich components of gunpowder can cause corrosion of the metals of the cartridge case, bullet and primer of loaded ammunition or the metals of the powder packaging if the latter is stored separately.
To avoid the accumulation of acidic decomposition products in the powder, stabilizers are added, the most popular of which are
Man has made many discoveries that were of great importance in one area or another of life. However, very few of these discoveries actually affected the course of history.
Gunpowder and its invention are precisely from this list of discoveries that contributed to the development of many areas of humanity.
Story
Background to the appearance of gunpowder
Scientists have debated for a long time about the time of its creation. Some argued that it was invented in Asian countries, while others, on the contrary, disagree and prove the opposite, that gunpowder was invented in Europe, and from there it came to Asia.
Everyone agrees that China is the birthplace of gunpowder.
The existing manuscripts speak of noisy holidays that were held in the Middle Kingdom with very loud explosions that were not familiar to Europeans. Of course, it was not gunpowder, but bamboo seeds, which burst with loud noise when heated. Such explosions made Tibetan monks think about the practical application of such things.
History of invention
Now it is no longer possible to determine with an accuracy of one year the time of the invention of gunpowder by the Chinese, however, according to manuscripts that have survived to this day, there is an opinion that in the middle of the 6th century, the inhabitants of the Celestial Empire also knew the composition of substances with the help of which fire with a bright flame could be obtained. The Taoist monks advanced the furthest towards the invention of gunpowder, who eventually invented gunpowder.
Thanks to the found work of monks, which was dated back to the 9th century, which contains lists of all certain “elixirs” and how to use them.
Much attention was paid to the text, which indicated the prepared composition, which unexpectedly ignited right after production and caused burns to the monks.
If the fire was not put out immediately, the alchemist’s house would burn to the ground.
Thanks to such information, discussions about the place and time of the invention of gunpowder were ended. Well, I must say that after the invention of gunpowder, it only burned, but did not explode.
The first composition of gunpowder
The composition of gunpowder required an exact ratio of all components. It took the monks another year to determine all the shares and components. As a result, a mixture was obtained that received the name “fire potion.” The potion contained molecules of coal, sulfur and saltpeter. There is very little saltpeter in nature, with the exception of the territories of China, where saltpeter can be found directly on the surface of the earth in a layer of several centimeters.
Gunpowder components:
Peaceful uses of gunpowder in China
When gunpowder was first invented, it was mainly used in the form of various sound effects or for colorful “fireworks” during entertainment events. However, local sages understood that the combat use of gunpowder was also possible.
China in those distant times was constantly at war with the nomads around it, and the invention of gunpowder was in the hands of military commanders.
Gunpowder: First military use by the Chinese
There are manuscripts by Chinese monks that claim the use of a “fire potion” for military purposes. The Chinese military surrounded the nomads and lured them into a mountainous area, where gunpowder charges were pre-installed and set on fire after the enemy’s campaign.
Strong explosions paralyzed the nomads, who fled in shame.
Having understood what gunpowder is and realizing its capabilities, the emperors of China supported the production of weapons using a fiery mixture, including catapults, powder balls, and various projectiles. Thanks to the use of gunpowder, the troops of the Chinese commanders did not know defeat and put the enemy to flight everywhere.

Gunpowder leaves China: Arabs and Mongols begin to make gunpowder
According to information received, around the 13th century, information about the composition and proportions for the manufacture of gunpowder was obtained by the Arabs; there is no exact information about how this was done. According to one legend, the Arabs massacred all the monks of the monastery and received a treatise. In the same century, the Arabs were able to build a cannon that could fire gunpowder shells.
"Greek Fire": Byzantine Gunpowder

Further information from the Arabs about gunpowder and its composition in Byzantium. By slightly changing the composition qualitatively and quantitatively, a recipe was obtained, which was called “Greek fire”. The first tests of this mixture were not long in coming.
During the defense of the city, cannons loaded with Greek fire were used. As a result, all the ships were destroyed by fire. Accurate information about the composition of “Greek fire” has not reached our times, but presumably it was used - sulfur, oil, saltpeter, resin and oils.
Gunpowder in Europe: who invented it?
For a long time, Roger Bacon was considered the culprit behind the appearance of gunpowder in Europe. In the mid-thirteenth century, he became the first European to describe in a book all the recipes for making gunpowder. But the book was encrypted, and it was not possible to use it.

If you want to know who invented gunpowder in Europe, then the answer to your question is the story of Berthold Schwartz. He was a monk and practiced alchemy for the benefit of his Franciscan Order. At the beginning of the fourteenth century he worked to determine the proportions of the substance from coal, sulfur and saltpeter. After much experimentation, he managed to grind the necessary components in a mortar in a proportion sufficient to cause an explosion.
The blast wave almost sent the monk to the next world.
The invention marked the beginning of the era of firearms.
The first model of the “shooting mortar” was developed by the same Schwartz, for which he was sent to prison in order to not disclose the secret. But the monk was kidnapped and secretly transported to Germany, where he continued his experiments in improving firearms.
How the inquisitive monk ended his life is still unknown. According to one version, he was blown up on a barrel of gunpowder; according to another, he died safely at a very old age. Be that as it may, gunpowder gave the Europeans great opportunities, which they did not fail to take advantage of.
The appearance of gunpowder in Rus'
There is no exact answer about the origin of gunpowder in Rus'. There are many stories, but the most plausible is considered to be that the composition of the gunpowder was provided by the Byzantines. For the first time, gunpowder was used in a firearm when defending Moscow from a raid by the troops of the Golden Horde. Such a gun did not incapacitate the enemy’s manpower, but made it possible to frighten horses and sow panic in the ranks of the Golden Horde.

Smokeless powder recipe: who invented it?

Approaching more modern centuries, let's say that the 19th century is a time of improvement of gunpowder. One of the interesting improvements is the invention of pyroxylin powder, which has a solid structure, by the Frenchman Viel. Its first use was appreciated by representatives of the defense department.
The point is that the gunpowder burned without smoke, leaving no traces.
A little later, inventor Alfred Nobel announced the possibility of using nitroglycerin gunpowder in the production of projectiles. After these inventions, gunpowder was only improved and its characteristics improved.
Types of gunpowder
The following types of gunpowder are used in the classification:
- mixed(the so-called black powder (black powder));
- nitrocellulose(respectively, smokeless).
It may be a discovery for many, but solid rocket fuel used in spacecraft and rocket engines is nothing more than the most powerful gunpowder. Nitrocellulose powders consist of nitrocellulose and a plasticizer. In addition to these parts, various additives are mixed into the mixture.
The storage conditions of gunpowder are of great importance. If the gunpowder is found beyond the possible storage period or the technological storage conditions are not met, irreversible chemical decomposition and deterioration of its properties are possible. Therefore, storage is of great importance in the life of gunpowder, otherwise an explosion may occur.
Black powder
Black powder is produced on the territory of the Russian Federation in accordance with the requirements of GOST-1028-79.
Nowadays, the production of smoky or black powder is regulated and complies with regulatory requirements and rules.
The types of gunpowder are divided into:
- grainy;
- powder powder.
Black powder consists of potassium nitrate, sulfur and charcoal.
- potassium nitrate oxidizes, allowing to burn at a rapid rate.
- charcoal is a fuel (which is oxidized by potassium nitrate).
- sulfur- a component that is necessary to ensure ignition. The requirements for the proportions of black powder grades are different in different countries, but the differences are not large.

The shape of granular grades of gunpowder after production resembles grain. Production consists of five stages:
- Grind to powder;
- Mixing;
- Pressed onto discs;
- Grain crushing occurs;
- The grains are polished.
The best grades of gunpowder burn better if all components are completely crushed and thoroughly mixed, even the output shape of the granules is important. The combustion efficiency of black powder is largely related to the fineness of grinding of the components, the completeness of mixing and the shape of the finished grains.
Types of black powders (% composition KNO 3, S, C.):
- corded (for fire cords) (77%, 12%, 11%);
- rifle (for igniters for charges of nitrocellulose powders and mixed solid fuels, as well as for expelling charges in incendiary and illuminating shells);
- coarse-grained (for igniters);
- slow-burning (for intensifiers and moderators in tubes and fuses);
- mine (for blasting) (75%, 10%, 15%);
- hunting (76%, 9%, 15%);
- sports.
When handling black powder, you must take precautions and keep the gunpowder away from an open source of fire, as it ignites easily; a flash at a temperature of 290-300 °C is sufficient for this.
There are high requirements for packaging. It must be sealed and black powder must be stored separately from the rest. Very picky about moisture content. If the moisture content is more than 2.2%, this powder is very difficult to ignite.
Before the beginning of the 20th century, black powder was invented for use in firing weapons and in various throwing grenades. Now used in the production of fireworks.
Varieties of gunpowder
Aluminum grades of gunpowder have found their use in the pyrotechnic industry. The basis is potassium/sodium nitrate (needed as an oxidizer), aluminum powder (this is flammable) and sulfur, reduced to the state of powder and mixed together. Due to the large release of light during combustion and the speed of combustion, it is used in explosive elements and flash compositions (producing a flash).
Proportions (saltpeter: aluminum: sulfur):
- bright flash - 57:28:15;
- explosion - 50:25:25.
Gunpowder is not afraid of moisture and does not change its flowability, but it can get very dirty.

Classification of gunpowders
This is a smokeless powder that was developed in modern times. Unlike black powder, nitrocellulose has a high efficiency. And there is no smoke that the arrow can give off.
In turn, nitrocellulose powders, due to the complexity of their composition and wide application, can be divided into:
- pyroxylin;
- ballistic;
- cordite.
Smokeless powder is a powder that is used in modern types of weapons and various explosive products. It is used as a detonator.
Pyroxylin
The composition of pyroxylin powders usually includes 91-96% pyroxylin, 1.2-5% volatile substances (alcohol, ether and water), 1.0-1.5% stabilizer (diphenylamine, centralite) to increase storage stability, 2- 6% phlegmatizer to slow down the combustion of the outer layers of powder grains and 0.2-0.3% graphite as additives.
Pyroxylin powders are produced in the form of plates, ribbons, rings, tubes and grains with one or more channels; The main uses are pistols, machine guns, cannons, and mortars.
The production of such gunpowder consists of the following stages:
- Dissolution (plasticization) of pyroxylin;
- Composition pressing;
- Cut from a mass with various shapes of gunpowder elements;
- Solvent removal.

Ballistic
Ballistic powders are gunpowders of artificial origin. The largest percentage has the following components:
- nitrocellulose;
- non-removable plasticizer.
Due to the presence of exactly 2 components, experts call this type of gunpowder 2-basic.
If there are changes in the percentage of gunpowder plasticizer content, they are divided into:
- nitroglycerin;
- diglycol.
The structure of the composition of ballistic powders is as follows:
- 40-60% colloxylin (nitrocellulose with a nitrogen content of less than 12.2%);
- 30-55% nitroglycerin (nitroglycerin powders) or diethylene glycol dinitrate (diglycol powders) or a mixture thereof;
Also included are various components that have a small percentage of content, but they are extremely important:
- dinitrotoluene– necessary to be able to control the combustion temperature;
- stabilizers(diphenylamine, centralite);
- Vaseline oil, camphor and other additives;
- Fine metal can also be added to ballistic powders(an alloy of aluminum and magnesium) to increase the temperature and energy of combustion products, such gunpowder is called metallized.
Continuous technological scheme for the production of powder mass of high-energy ballistic powders
 1 – agitator; 2 – mass pump; 3 – volumetric pulse dispenser; 4 – bulk components dispenser; 5 – supply container; 6 – supply tank; 7 – gear pump; 8 – APR; 9 – injector;
1 – agitator; 2 – mass pump; 3 – volumetric pulse dispenser; 4 – bulk components dispenser; 5 – supply container; 6 – supply tank; 7 – gear pump; 8 – APR; 9 – injector; 10 – container; 11 – passivator; 12 – water repellent; 13 – solvent; 14 – mixer; 15 – intermediate mixer; 16 – mixer of common batches
The appearance of the manufactured gunpowder is in the form of tubes, checkers, plates, rings and ribbons. Gunpowder is used for military purposes, and according to their application they are divided:
- rocket(for charges for rocket engines and gas generators);
- artillery(for propellant charges for artillery pieces);
- mortar(for propellant charges for mortars).
Compared to pyroxylin powders, ballistic gunpowders are characterized by lower hygroscopicity, faster production, the ability to produce large charges (up to 0.8 meters in diameter), high mechanical strength and flexibility due to the use of a plasticizer.
The disadvantages of ballistic powders compared to pyroxylin powders include:
- Great danger in production due to the presence in their composition of a powerful explosive - nitroglycerin, which is very sensitive to external influences, as well as the inability to obtain charges with a diameter of more than 0.8 m, in contrast to mixed gunpowders based on synthetic polymers;
- Complexity of the production process ballistic powders, which involves mixing the components in warm water in order to distribute them evenly, squeezing out the water and repeated rolling on hot rollers. This removes water and plasticizes the cellulose nitrate, which takes on the appearance of a horn-like sheet. Next, the gunpowder is pressed through dies or rolled into thin sheets and cut.
Cordite
Cordite powders contain high-nitrogen pyroxylin, a removable (alcohol-ether mixture, acetone) and non-removable (nitroglycerin) plasticizer. This brings the production technology of these gunpowders closer to the production of pyroxylin gunpowder.
The advantage of cordites is greater power, but they cause increased burning of the barrels due to the higher temperature of the combustion products.

Solid rocket fuel
Synthetic polymer-based mixed propellant (solid rocket fuel) contains approximately:
- 50-60% oxidizing agent, usually ammonium perchlorate;
- 10-20% plasticized polymer binder;
- 10-20% fine aluminum powder and other additives.
This direction of powder making first appeared in Germany in the 30-40s of the 20th century; after the end of the war, the active development of such fuels began in the USA, and in the early 50s - in the USSR. The main advantages over ballistic gunpowder, which attracted a lot of attention to them, were:
- high specific thrust of rocket engines using such fuel;
- the ability to create charges of any shape and size;
- high deformation and mechanical properties of the compositions;
- the ability to regulate the burning rate over a wide range.
These properties of gunpowder made it possible to create strategic missiles with a range of more than 10,000 km. Using ballistic gunpowder, S.P. Korolev, together with gunpowder makers, managed to create a rocket with a maximum range of 2,000 km.
But mixed solid fuels have significant disadvantages compared to nitrocellulose powders: the very high cost of their production, the duration of the charge production cycle (up to several months), the complexity of disposal, the release of hydrochloric acid into the atmosphere during the combustion of ammonium perchlorate.
 The new gunpowder is solid rocket fuel.
The new gunpowder is solid rocket fuel. Powder combustion and its regulation
Combustion in parallel layers, which does not turn into an explosion, is caused by the transfer of heat from layer to layer and is achieved by manufacturing fairly monolithic powder elements, free of cracks.
The burning rate of gunpowder depends on pressure according to a power law, increasing with increasing pressure, so you should not focus on the burning rate of gunpowder at atmospheric pressure when assessing its characteristics.
Regulating the burning rate of gunpowder is a very difficult task and is solved by using various combustion catalysts in the powder composition. Combustion in parallel layers allows you to regulate the rate of gas formation.
The gas formation of gunpowder depends on the size of the surface of the charge and its burning rate.

The surface area of the powder elements is determined by their shape, geometric dimensions and can increase or decrease during the combustion process. Such combustion is called progressive or digressive, respectively.
To obtain a constant rate of gas formation or its change according to a certain law, individual sections of charges (for example, missiles) are covered with a layer of non-combustible materials (armor).
The burning rate of gunpowder depends on its composition, initial temperature and pressure.
Characteristics of gunpowder
The characteristics of gunpowder are based on parameters such as:
- heat of combustion Q- the amount of heat released during complete combustion of 1 kilogram of gunpowder;
- volume of gaseous products V released during the combustion of 1 kilogram of gunpowder (determined after bringing the gases to normal conditions);
- gas temperature T, determined by combustion of gunpowder under conditions of constant volume and absence of heat losses;
- powder density ρ;
- gunpowder strength f- the work that could be done by 1 kilogram of powder gases, expanding when heated by T degrees at normal atmospheric pressure.
Characteristics of nitro powders
Non-military use
The ultimate main purpose of gunpowder is military purposes and use for the destruction of enemy targets. However, the composition of Sokol gunpowder allows its use for peaceful purposes, such as fireworks, construction tools (construction pistols, punches), and in the field of pyrotechnics - squibs. The characteristics of Bars gunpowder are more suitable for use in sports shooting.

5. Smokeless explosive components
Pyroxylin
Since Napoleon's time, military commanders had complained of their inability to issue orders in battle due to the heavy smoke caused by the gunpowder used in guns.
A major breakthrough was made with the invention of pyroxylin, a material based on nitrocellulose. It has found wide application in artillery.
However, pyroxylin had a number of significant disadvantages. Pyroxylin was more powerful than black powder, but at the same time less stable, making it unsuitable for use with small firearms - not only because it was more dangerous in the field, but also because of increased wear and tear on the weapon. A weapon that could fire thousands of times with ordinary gunpowder became unusable after several hundred shots with more powerful gunpowder. There have also been many explosions in pyroxylin factories due to neglect of its instability and means of stabilization.
For these reasons, the use of pyroxylin was suspended for more than twenty years, until people learned to “tame” it. It was not until 1880 that pyroxylin became a viable explosive.
White powder
In 1884, Paul Viel invented a smokeless gunpowder called Poudre B, which was based on gelatinized gunpowder mixed with ether and alcohol, further forming the gunpowder elements and then drying the gunpowder grains.
The final explosive, which today is called nitrocellulose, contains a slightly smaller amount of nitrogen than pyroxylin, so it is more easily gelled by the alcohol-ether mixture. The great advantage of this gunpowder was that, unlike pyroxylin, it burns in layers, which made its ballistic properties predictable.
Viel gunpowder revolutionized the world of small arms for several reasons:
- There was virtually no smoke anymore, whereas previously, after several shots using black powder, the soldier's field of vision was greatly reduced due to clouds of smoke, which could only be corrected by a strong wind. In addition, the shooter's position was not indicated by a puff of smoke from the rifle.
- Poudre B gave a higher bullet velocity, which meant a straighter trajectory, which increased accuracy and range; The firing range reached 1000 meters.
- Since Poudre B was three times more powerful than black powder, much less of it was needed. Ammunition was made lighter, allowing troops to carry more ammunition for the same weight.
- The cartridges worked even when wet. Ammunition based on black powder had to be stored in a dry place, so they were always carried in closed packages that prevented moisture from entering.
Vieille gunpowder was used in the Lebel rifle, which was immediately adopted by the French Army to take full advantage of the new gunpowder over black gunpowder. Other European countries rushed to follow the example of the French and also switched to their derivatives of the Poudre B. The first were Germany and Austria, which introduced the new weapon in 1888.
Ballistitis
During this time in 1887 in Great Britain, Alfred Nobel developed a smokeless gunpowder called ballistite.
Cordite
Ballistite was modified by Frederick Abel and James Dewar into a new compound called cordite. After this, a “patent war” began between Nobel and the inventors of cordite over obtaining British patents.
In 1890, Maxim Hudson received a patent for smokeless gunpowder in the United States.
These new explosives were more stable and therefore safer to handle than Poudre B and, importantly, more powerful.
Gelatin powder
Source
Ivan Platonovich Grave professor of the Mikhailovsky Artillery Academy, colonel, in 1916 improved the French invention: he obtained smokeless gunpowder on a different basis on a non-volatile solvent, colloidal, or gelatinous, gunpowder. It was easy to mold and even turn on a lathe. Gelatin powder was used in checkers.
Grave received a patent for this invention in 1926 in another country - Soviet Russia. He received 9 patents, but as a nobleman he was forbidden to develop rockets and he took up science. The Main Artillery Directorate confirms his authorship in the development of gunpowder and shells for the Katyusha.
If you find an error on a page, select it and press Ctrl + Enter
Smokeless powder: a failed experience of "fire in the palm of your hand"
The first explosive that man became acquainted with was black (smoky) gunpowder: it was known in China, starting around the 10th century AD. There is an opinion that black powder for a long time served only as idle entertainment, and it took centuries for it to begin to be used in military affairs. In fact, this is not so; Chinese military leaders quickly realized that gunpowder is not only entertainment: it can be used to make effective weapons. Here's a quote:
In 1044, Emperor Renzong received a report “On the Fundamentals of Military Affairs” from one of his confidants. The text contained two recipes for making a "fire potion" suitable for use in incendiary bombs that could be thrown by siege engines. The third mixture was intended as fuel for poisonous smoke bombs. The proportion of nitrate in all three mixtures was low, meaning that they were designed to burn rapidly rather than explode. These were the world's first applied gunpowder formulas.
Over many centuries of almost continuous wars, the composition of black powder changed greatly, but the components remained the same (potassium nitrate, sulfur, charcoal). Black powder had many disadvantages that hampered the development of firearms and warfare. For example, in the era of the Napoleonic Wars, after several volleys of rifles and cannons, the battlefield was clouded with thick smoke, which greatly interfered with targeted shooting and army control. - It was simply impossible to see anything. The army could be on the verge of defeat or just moments away from victory, and the commander who was nearby could not see it. And in any case, it was difficult to convey orders to subordinates.
Black powder was replaced by smokeless powder. The basis of smokeless powder is nitrocellulose. Nowadays, several percent of nitroglycerin is added to nitrocellulose (so-called dibasic gunpowder - they are used in small arms). In addition to nitroglycerin, nitroguanidine is added to tribasic powders. Such gunpowder is used in artillery. In addition to the main components, various additives are added to smokeless powder to improve its properties.
Both black and smokeless powder are capable of exploding; for example, black powder was used in explosives for a long time until it was replaced by nitroglycerin and dynamite. Pyroxylin (fully nitrated cellulose) was used for some time to fill shells. To prevent gunpowder from burning, but from exploding, it must be ignited in a closed volume. Another option is to ignite a large mass of gunpowder, poured compactly. How big depends greatly on many factors (brand of gunpowder, packing density, size of granules, shape of the pile, etc.). I remember my friend set fire to a matchbox with Bars gunpowder, which he removed from mounting cartridges (he also added magnesium powder to the gunpowder) - this was enough for the combustion to turn into an explosion. As a result, the entire T-shirt was full of holes from hot particles.
However, the main task of gunpowder is to burn quickly and evenly. This is exactly the result they are trying to achieve in their production. To do this, not only a special chemical composition is selected, but gunpowder is produced in the form of grains and granules of the desired shape and size.
Granular black powder burns so quickly that you can burn a small amount of black powder on the palm of your hand without getting burned (take no more than a gram, preferably less). Just before trying the experiment on your hand, it is advisable to burn a small pile of black powder on paper. High-quality gunpowder should burn without leaving traces ("missing" or black areas). If the paper is not damaged, then most likely your skin will not be affected either.
Is it possible to conduct such an experiment with smokeless powder? - After all, the basis of smokeless powder is nitrocellulose. Nitrocellulose in the form of nitrated cotton wool can be burned on the palm of your hand without getting a burn and without feeling pain (or almost without feeling it).
I knew the answer to this question in advance - NO. Smokeless powder burns too slowly and will obviously cause burns. To draw such a conclusion, it was enough to watch how a “column” of artillery gunpowder burns (I observed it back in school - more than twenty years ago). However, I tried. He poured a bunch of large granules of gunpowder from DShK machine gun cartridges into his palm and set it on fire with a lighter. The pain was such that after 2 seconds the flame had to be extinguished (by squeezing my palm). A burn of approximately a square centimeter was left on the skin; it took more than a month to heal. After the flame went out, at first I did not feel any pain, but later this burn caused a lot of discomfort.
__________________________________________________
Mendeleev's smokeless powder
It is believed that Mendeleev invented 40-proof vodka - he diluted the alcohol with water in the appropriate proportion. In fact, in 1865 he defended his doctoral dissertation “Discourse on the combination of alcohol with water.” Forty-proof vodka was produced before his dissertation. Mendeleev's merit is that he compiled the table “Values of the specific gravities of aqueous solutions of alcohol”; it was his calculations that were used in the production of alcoholic beverages.
In his rich biography there is another fact that few people know; at one time it was kept in the strictest confidence - the invention of smokeless gunpowder for artillery. In 1890, Naval Minister N.M. Chikhachev approached him with a proposal to take part in the development of types of smokeless gunpowder for firing artillery guns in the navy. Such gunpowder was already in service with Great Britain and France. The basis of most smokeless gunpowder was pyroxylin, a product of processing cotton wool with a mixture of nitric and sulfuric acids. However, information about the technology for creating pyroxylin was kept in the strictest confidence. Mendeleev took up the solution to this problem.
Soon he and two other specialists were sent abroad, to London, then to Paris. In London, Mendeleev had many acquaintances among chemist scientists. He visited different laboratories and was even taken to shooting range. But the technology for making smokeless gunpowder remained a secret. In Paris the situation repeated itself. He attended a meeting of the Paris Academy of Sciences and received samples of smokeless gunpowder. But how to organize the production of smokeless powder suitable for artillery shooting? What did Mendeleev do?
There is a version that Mendeleev settled down near one of the gunpowder factories in Paris and began to observe the arrival of freight cars with various raw materials along the railway line: nitrogen, sulfuric acid, alcohol, oxygen and their exit with finished products - shells. After studying statistical data, he came to the conclusion of what proportions of explosives French smokeless powder could consist of.
Soon the secret report landed on the minister’s desk. Mendeleev was invited to work at the Marine Scientific and Technical Laboratory, where he conducted his experiments. And in the same 1890, he discovered pyrocollodium, which he proposed as a smokeless gunpowder, superior to foreign pyroxylin. Firing of 47 mm caliber cannons carried out in 1892 showed the remarkable properties of pyrocollodium. But bureaucratic leapfrog intervened, and Mendeleev’s pyro-collodion gunpowder was not adopted by the land department. The saddest thing is that the manufacturing process was not carefully classified, and soon pyrocollodion gunpowder was at the disposal of Western countries.
After the death of the scientist during the First World War, Russia was forced to purchase from the United States a huge amount of smokeless gunpowder, which was, in fact, Mendeleev’s pyrocollodion gunpowder.
 Mariupol State Humanitarian University Mggu Mariupol State Humanitarian University
Mariupol State Humanitarian University Mggu Mariupol State Humanitarian University Nine muses of Ancient Greece: what inspired the creators and what gifts did they possess?
Nine muses of Ancient Greece: what inspired the creators and what gifts did they possess? What is fire and why does it burn?
What is fire and why does it burn? Deferred tax liabilities example Deferred tax liabilities are accounted for as part of
Deferred tax liabilities example Deferred tax liabilities are accounted for as part of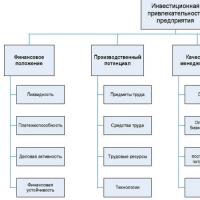 How to increase the investment attractiveness of an organization Measures to increase the investment attractiveness of an enterprise
How to increase the investment attractiveness of an organization Measures to increase the investment attractiveness of an enterprise How can we now determine the initial cost of fixed assets according to IFRS?
How can we now determine the initial cost of fixed assets according to IFRS?