What electric charge do electrons have? What electric charges do electrons and neutrons have? Examples of problem solving
Until the beginning of the 20th century, scientists believed that an atom was the smallest indivisible particle of matter, but this turned out to be wrong. In fact, at the center of the atom is its nucleus with positively charged protons and neutral neutrons, and negatively charged electrons rotate in orbitals around the nucleus (this model of the atom was proposed in 1911 by E. Rutherford). It is noteworthy that the masses of protons and neutrons are almost equal, but the mass of an electron is about 2000 times less.
Although an atom contains both positively and negatively charged particles, its charge is neutral, because an atom has the same number of protons and electrons, and differently charged particles neutralize each other.
Later, scientists found out that electrons and protons have the same amount of charge, equal to 1.6 10 -19 C (C is a coulomb, a unit of electric charge in the SI system.
Have you ever thought about the question - what number of electrons corresponds to a charge of 1 C?
1/(1.6·10 -19) = 6.25·10 18 electrons
Electric power
Electric charges influence each other, which manifests itself in the form electric force.
If a body has an excess of electrons, it will have a total negative electrical charge, and vice versa - if there is a deficiency of electrons, the body will have a total positive charge.
By analogy with magnetic forces, when like-charged poles repel and oppositely charged poles attract, electric charges behave in a similar way. However, in physics it is not enough to simply talk about the polarity of an electric charge; its numerical value is important.
To find out the magnitude of the force acting between charged bodies, it is necessary to know not only the magnitude of the charges, but also the distance between them. The force of universal gravitation has already been considered previously: F = (Gm 1 m 2)/R 2
- m 1, m 2- body masses;
- R- the distance between the centers of the bodies;
- G = 6.67 10 -11 Nm 2 /kg- universal gravitational constant.
As a result of laboratory experiments, physicists derived a similar formula for the force of interaction of electric charges, which was called Coulomb's law:
F = kq 1 q 2 /r 2
- q 1, q 2 - interacting charges, measured in C;
- r is the distance between charges;
- k - proportionality coefficient ( SI: k=8.99·10 9 Nm 2 Cl 2; SSSE: k=1).
- k=1/(4πε 0).
- ε 0 ≈8.85·10 -12 C 2 N -1 m -2 - electrical constant.
According to Coulomb's law, if two charges have the same sign, then the force F acting between them is positive (the charges repel each other); if the charges have opposite signs, the acting force is negative (charges attract each other).
How enormous the force of a charge of 1 C is can be judged using Coulomb's law. For example, if we assume that two charges, each 1 C, are spaced at a distance of 10 meters from each other, then they will repel each other with force:
F = kq 1 q 2 /r 2 F = (8.99 10 9) 1 1/(10 2) = -8.99 10 7 N
This is a fairly large force, roughly comparable to a mass of 5600 tons.
Let's now use Coulomb's law to find out at what linear speed the electron rotates in a hydrogen atom, assuming that it moves in a circular orbit.
According to Coulomb's law, the electrostatic force acting on an electron can be equated to the centripetal force:
F = kq 1 q 2 /r 2 = mv 2 /r
Taking into account the fact that the mass of the electron is 9.1·10 -31 kg, and the radius of its orbit = 5.29·10 -11 m, we obtain the value 8.22·10 -8 N.
Now we can find the linear speed of the electron:
8.22·10 -8 = (9.1·10 -31)v 2 /(5.29·10 -11) v = 2.19·10 6 m/s
Thus, the electron of the hydrogen atom rotates around its center at a speed of approximately 7.88 million km/h.
DEFINITION
Proton called a stable particle belonging to the class of hadrons, which is the nucleus of a hydrogen atom.
Scientists disagree on which scientific event should be considered the discovery of the proton. An important role in the discovery of the proton was played by:
- creation of a planetary model of the atom by E. Rutherford;
- discovery of isotopes by F. Soddy, J. Thomson, F. Aston;
- observations of the behavior of the nuclei of hydrogen atoms when they are knocked out by alpha particles from nitrogen nuclei by E. Rutherford.
The first photographs of proton tracks were obtained by P. Blackett in a cloud chamber while studying the processes of artificial transformation of elements. Blackett studied the process of capture of alpha particles by nitrogen nuclei. In this process, a proton was emitted and the nitrogen nucleus was converted into an isotope of oxygen.
Protons, together with neutrons, are part of the nuclei of all chemical elements. The number of protons in the nucleus determines the atomic number of the element in the periodic table D.I. Mendeleev.
A proton is a positively charged particle. Its charge is equal in magnitude to the elementary charge, that is, the value of the electron charge. The charge of a proton is often denoted as , then we can write that:
It is currently believed that the proton is not an elementary particle. It has a complex structure and consists of two u-quarks and one d-quark. The electric charge of a u-quark () is positive and it is equal to
The electric charge of a d-quark () is negative and equal to:
Quarks connect the exchange of gluons, which are field quanta; they endure strong interaction. The fact that protons have several point scattering centers in their structure is confirmed by experiments on the scattering of electrons by protons.
The proton has a finite size, which scientists are still arguing about. Currently, the proton is represented as a cloud that has a blurred boundary. Such a boundary consists of constantly emerging and annihilating virtual particles. But in most simple problems, a proton can, of course, be considered a point charge. The rest mass of a proton () is approximately equal to:
The mass of a proton is 1836 times greater than the mass of an electron.
Protons take part in all fundamental interactions: strong interactions unite protons and neutrons into nuclei, electrons and protons join together in atoms using electromagnetic interactions. As a weak interaction, we can cite, for example, the beta decay of a neutron (n):
where p is proton; — electron; - antineutrino.
Proton decay has not yet been obtained. This is one of the important modern problems of physics, since this discovery would be a significant step in understanding the unity of the forces of nature.
Examples of problem solving
EXAMPLE 1
| Exercise | The nuclei of the sodium atom are bombarded with protons. What is the force of electrostatic repulsion of a proton from the nucleus of an atom if the proton is at a distance |
| Solution | As a basis for solving the problem, we will take Coulomb’s law, which can be written for our problem (assuming the particles are point particles) as follows:
where F is the force of electrostatic interaction of charged particles; Cl is the proton charge; - charge of the nucleus of the sodium atom; - dielectric constant of vacuum; - electrical constant. Using the data we have, we can calculate the required repulsive force: |
| Answer | N |
EXAMPLE 2
| Exercise | Considering the simplest model of the hydrogen atom, it is believed that the electron moves in a circular orbit around the proton (the nucleus of the hydrogen atom). What is the speed of an electron if the radius of its orbit is m? |
| Solution | Let's consider the forces (Fig. 1) that act on an electron moving in a circle. This is the force of attraction from the proton. According to Coulomb's law, we write that its value is equal to ():
where =— electron charge; |
If you rub a glass rod on a sheet of paper, the rod will acquire the ability to attract leaves of the “sultan” (see Fig. 1.1), fluff, and thin streams of water. When you comb dry hair with a plastic comb, the hair is attracted to the comb. In these simple examples we encounter the manifestation of forces that are called electrical.
Rice. 1.1. Attracting the leaves of the “sultan” with an electrified glass rod.
Bodies or particles that act on surrounding objects with electrical forces are called charged or electrified. For example, the glass rod mentioned above, after being rubbed on a piece of paper, becomes electrified.
Particles have an electrical charge if they interact with each other through electrical forces. Electrical forces decrease with increasing distance between particles. Electrical forces are many times greater than the forces of universal gravity.
Electric charge is a physical quantity that determines the intensity of electromagnetic interactions. Electromagnetic interactions are interactions between charged particles or bodies.
Electric charges are divided into positive and negative. Stable elementary particles have a positive charge - protons And positrons, as well as ions of metal atoms, etc. Stable negative charge carriers are electron And antiproton.
There are electrically uncharged particles, that is, neutral ones: neutron, neutrino. These particles do not participate in electrical interactions, since their electric charge is zero. There are particles without an electric charge, but an electric charge does not exist without a particle.
Positive charges appear on glass rubbed with silk. Ebonite rubbed on fur has negative charges. Particles repel when charges have the same signs ( charges of the same name), and with different signs ( unlike charges) particles are attracted.
All bodies are made of atoms. Atoms consist of a positively charged atomic nucleus and negatively charged electrons that move around the atomic nucleus. The atomic nucleus consists of positively charged protons and neutral particles - neutrons. The charges in an atom are distributed in such a way that the atom as a whole is neutral, that is, the sum of the positive and negative charges in the atom is zero.
Electrons and protons are part of any substance and are the smallest stable elementary particles. These particles can exist in a free state for an unlimited time. The electric charge of an electron and a proton is called the elementary charge.
Elementary charge- this is the minimum charge that all charged elementary particles have. The electric charge of a proton is equal in absolute value to the charge of an electron:
E = 1.6021892(46) * 10 -19 C The magnitude of any charge is a multiple in absolute value of the elementary charge, that is, the charge of the electron. Electron translated from Greek electron - amber, proton - from Greek protos - first, neutron from Latin neutrum - neither one nor the other.
Conductors and dielectrics
Electric charges can move. Substances in which electric charges can move freely are called conductors. Good conductors are all metals (conductors of the first kind), aqueous solutions of salts and acids - electrolytes(type II conductors), as well as hot gases and other substances. The human body is also a conductor. Conductors have high electrical conductivity, that is, they conduct electric current well.
Substances in which electric charges cannot move freely are called dielectrics(from English dielectric, from Greek dia - through, through and English electric - electric). These substances are also called insulators. The electrical conductivity of dielectrics is very low compared to metals. Good insulators are porcelain, glass, amber, ebonite, rubber, silk, gases at room temperatures and other substances.
The division into conductors and insulators is arbitrary, since conductivity depends on various factors, including temperature. For example, glass insulates well only in dry air and becomes a poor insulator when the air humidity is high.
Conductors and dielectrics play a huge role in modern applications of electricity.
What is an atom? Translated into Russian, atom means indivisible. For a long time no one could refute this statement. Finally, at the end of the 19th century, it was proven that the atom is divided into smaller particles, the main ones being electrons, protons and neutrons.
When studying these particles, it turned out that protons and electrons have electric charges, and their charges are equal in magnitude, but opposite in sign. The charge of an electron refers to that electricity that is called negative, and the charge of a proton refers to that that is called positive.
The mass of an electron is approximately 1840 times less than the mass of a proton.
Since electrons and protons are electrically charged, they obey the law on the interaction of electric charges: like charges repel (proton with proton and electron with electron), and unlike charges attract (proton with electron).
Neutron- the third particle in the atom, the mass is equal to the proton, but the neutron does not have an electric charge. It is said to be electrically neutral, hence its name - neutron.
As mentioned above, the atom has a very complex structure, but for the first time we can limit ourselves to the following simplified idea of its structure.
At the center of the atom is the nucleus, it consists of protons and neutrons, therefore it is positively charged. Electrons revolve around the nucleus at an impressive distance, hundreds of thousands of times greater than its size.
Since each atom has the same number of electrons as the number of protons, it is considered electrically neutral.
The simplest atom in structure is the hydrogen atom; its nucleus consists of one proton, around which one electron rotates.
Atoms of various substances differ from one another in the number of protons, neutrons and electrons.
What is an ion? If somehow an atom loses one or more electrons, it will become positively charged, such an atom will be called a positive ion, and if the atom gains one or more electrons, it will be called a negative ion, because it will be negatively charged.
Electric field. Scientists have established the existence of a special type of matter - a field. Around electric charges there is also a field called electric. A characteristic feature of this field is the mechanical force acting on the electrical charges located in this field. Most often, the electric field is depicted in drawings in the form of arrows showing the direction in which a free positive charge would move under the influence of the forces of this field. These lines are also called power lines. In reality there are no lines.

Conductors and insulators. In different substances, electrons are bonded to their atoms in different ways, in some the bond is strong, in others it is not. Electrons that are poorly bonded to atoms and that can easily leave them are called free electrons. If in one of the points of a substance in which free electrons are present, an excess of them is created, and in another - a deficiency, then they, maintaining a chaotic movement, will begin to move with their entire mass to that point, the side where there are not enough electrons. This one-way movement will be called electric current. Substances that contain free electrons are called conductors of electric current. In other substances, for example mica, rubber, electrons, on the contrary, are very tightly bound to their atoms and under normal conditions will not be able to leave them; in such substances, current will never arise, which is why they are called non-conductors, or insulators.
1. Basic principles of molecular kinetic theory? 2. How is energy transferred from the Sun to the Earth? 3.Whichwill the substance feel hottest to the touch in hot weather?
E) Glass
4. How much heat will be released during the complete combustion of gasoline weighing 5 kg. The specific heat of combustion of gasoline is 4.6 * 10^7 J/kg.
5.What electric charges do an electron and a proton have?
1) Determine the current strength in a light bulb if an electric charge of 300 C passes through its filament in 10 minutes.2) What electric charge will pass through the ammeter in 3 minutes when the current in the circuit is 0.2 A?
3) When electric welding, the current reaches 200 A. How long does it take for a charge of 60,000 C to pass through the cross section of the electrode?
4) A charge of 600 C passed through the spiral of the electric stove in 2 minutes. What is the current strength in the spiral?
5) The current strength in the iron is 0.2 A. What electric charge will pass through its coil in 5 minutes?
6) How long will it take for a charge equal to 30 C to pass through the cross-section of the conductor at a current of 200 mA?
PLEASE HELP AA!! Determine the current strength in an electric lamp if an electric charge of 300 C passes through its filament in 10 minutesWhat electric charge will pass through the ammeter in 3 minutes when the current in the circuit is 0.2A?
4. We cannot see electrons moving in a metal conductor. We can judge the presence of electric current in a circuit by the effects of the current. Whichactions are not those caused by electric current? A) thermal; B) mechanical; C) magnetic; D) chemical. 5. In ancient times, it was assumed that both positive and negative electrical charges could move in all conductors. The movement of which particles in an electric field is taken to be the direction of the current? A) positive charges; B) electrons; C) neutrons; D) negative ions. 6. Ampere Andre Marie - French physicist and mathematician. He created the first theory that expressed the connection between electrical and magnetic phenomena. Ampere has a hypothesis about the nature of magnetism. And what concept did he introduce into physics for the first time? A) current strength; B) electric current; C) electron; D) electric charge. 7. The work done by the forces of the electric field that creates an electric current is called the work of the current. It depends on the current strength. But work does not depend on current strength alone. What other quantity does it depend on? A) voltage; B) power; C) amount of heat; D) speed. 8. To measure the voltage at the poles of a current source or at some section of the circuit, a device called a voltmeter is used. Many voltmeters are very similar in appearance to ammeters. To distinguish it from other devices, the letter V is placed on the scale. But how is a voltmeter connected to the circuit? A) in parallel; B) sequentially; C) strictly behind the battery; D) connected to an ammeter. 9. The dependence of the current on the properties of the conductor is explained by the fact that different conductors have different electrical resistance. What does resistance not depend on? A) from differences in the structure of the crystal lattice; B) by weight; C) on length; D) from the cross-sectional area. 10. There are two ways to connect conductors: parallel and series. It is very convenient to use parallel connections of consumers in everyday life and in technology. Which electrical quantity is the same for all conductors connected in parallel: A) current strength; B) voltage; C) time; D) resistance. 11. In 5 s of motion, a body travels a distance of 12.5 m. What distance will the body travel in 6 s of motion, if the body moves with constant acceleration? A) 25 m; B) 13 m; C) 36 m; D) 18 m. 12. A student traveled one third of the way by bus at a speed of 60 km/h, and another third of the way by bicycle at a speed of 20 km/h. The last third of the journey was covered at a speed of 5 km/h. Determine the average speed of movement. A) 30 km/h; B) 10 km/h; C) 283 km/h; D) 11.25 km/h. 13. The density of water is taken to be 1000 kg/m3, and the density of ice is 900 kg/m3. If an ice floe floats, protruding 50 m3 above the surface of the water, what is the volume of the entire ice floe? A) 100 m3; B) 200 m3; C) 150 m3; D) 500 m3. 14. Weights and () are attached to the ends of a thin rod of length L. The rod is suspended on a thread and located horizontally. Find the distance x from the mass m1 to the point of suspension of the thread. Neglect the mass of the rod. A) x = (L∙m2) / (m1 – m2); B) x = (L∙m2) / (m1 + m2); C) x = (L∙m1) / (m1 – m2); D) x = (L∙m1) / (m1 + m2). 15. Climbers climb to the top of the mountain. How does atmospheric pressure change as athletes move? A) will increase; B) will not change; C) there is no correct answer. D) will decrease;

 The story of the pilots who bombed Hiroshima and Nagasaki
The story of the pilots who bombed Hiroshima and Nagasaki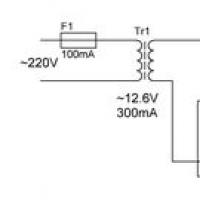 Smooth capacity charging: what to choose?
Smooth capacity charging: what to choose? Small Faculty of Mathematics
Small Faculty of Mathematics “Why do you dream about a round dance in a dream?
“Why do you dream about a round dance in a dream? Why do you dream about the church inside: interpretation of the meaning of the dream according to various dream books for men and women
Why do you dream about the church inside: interpretation of the meaning of the dream according to various dream books for men and women Dream interpretation of persimmon, why do you dream of persimmon in a dream to see Persimmon in a dream why
Dream interpretation of persimmon, why do you dream of persimmon in a dream to see Persimmon in a dream why Enchanted soul Meaning of karmic numbers
Enchanted soul Meaning of karmic numbers